Technology
We eliminate cancer cell by cell

Silver Nanocluster
We start with tiny silver nanoclusters—so small, they are invisible even under a microscope—and wrap them in a layer of DNA. This clever design keeps the particles safe ensuring they won’t harm healthy cells.
Entering the Nucleus
Light Activation
Controlled Impact
Disrupting DNA
Our approach tangles up the cancer cell’s DNA, making it impossible for the cell to survive. This process only affectes the cancer cells, reducing the side effects often seen with other treatments.
Why are we unique?
Unlike traditional treatments that affect both cancerous and healthy cells, our drug is activated only inside cancer cells. By using light to control when and where the treatment occurs, we only attack cancer cells, resulting in fewer side effects and better outcomes for patients.
A Payload to Targeting Therapies
Our drug acts as a universal payload for targeted therapeutics, whether guided by peptides, antibodies, or imaging techniques. This versatility enables both precision targeting and image-guided therapy across a broad spectrum of cancer types.
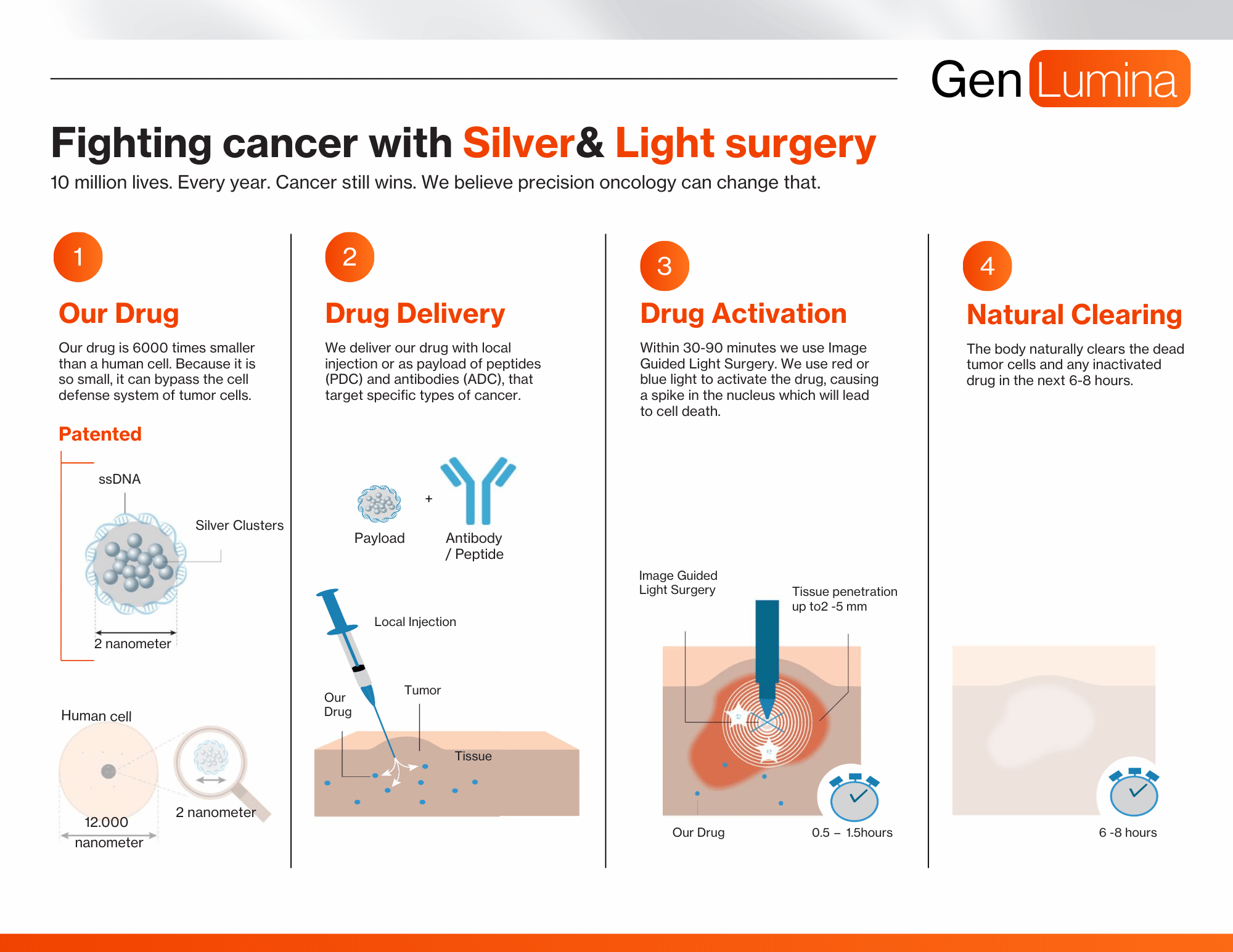
PDC
Peptide targeting cancer linked to our drug as payload
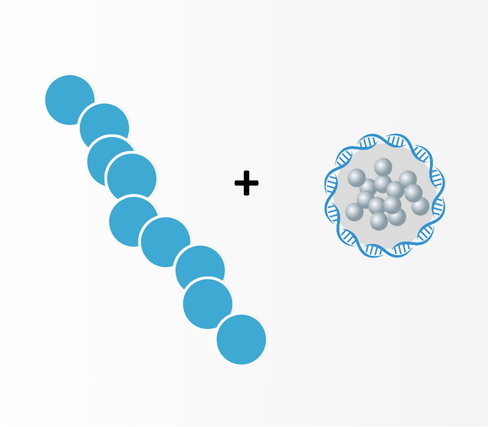
ADC
Antibody targeting cancer linked to our drug as payload
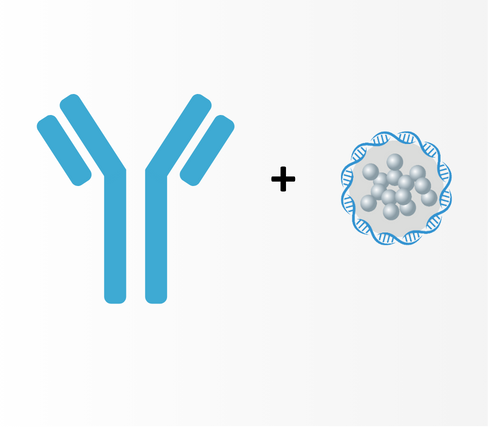
Standalone Drug
Image targeting cancer combined with local injection
Preclinical research
We performed pre-clinical studies in vitro and in vivo, and are in the middle of collecting more data regarding the efficacy, safety and toxicity of our drug.
In a lab
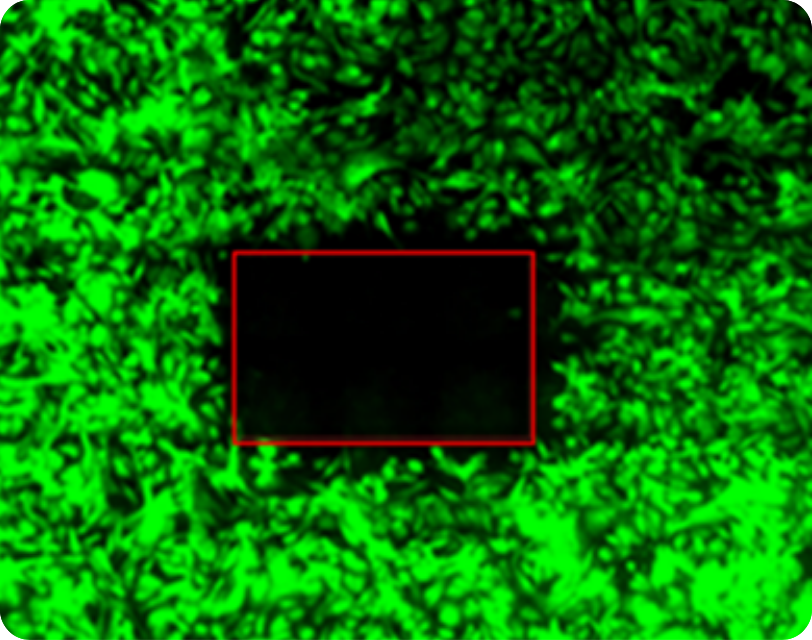
Lab tests show single cell precision & efficacy
In a mouse
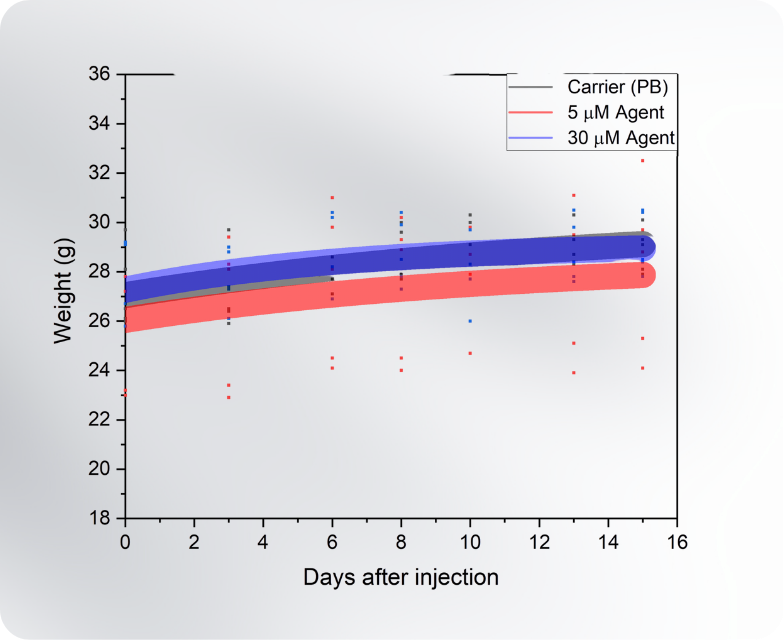
Mice show high safety ceiling (not activated)
FAQ
Given the availability of many clinically validated cytotoxic payloads, why is GenLumina’s payload relevant?
Resistance remains one of the central limitations of conventional chemotherapeutic payloads in ADCs. Tumors frequently develop biochemical escape mechanisms that enable survival despite continued treatment. Many of these resistance pathways arise because conventional payloads act through single enzymatic targets and depend on systemic exposure and intracellular processing for activity (ref 1). GenLumina’s SnB technology operates through a completely different mechanism. Upon localized light activation, the ultra-small, DNA-linked silver nanocluster engages directly with DNA at multiple sites within the nucleus, creating a physical and structural blockade rather than relying on inhibition of a specific biochemical pathway, leaving no straightforward mutational escape route usually found in resistance to chemotherapy.
This differentiated biology is complemented by a purpose-built conjugation architecture. Unlike conventional ADC payloads, which were not originally designed for conjugation and therefore require complex linker chemistries and multi-step manufacturing processes, SnB is engineered from the outset for targeted delivery. The payload incorporates a DNA bridge within its DNA shell with an attachment point at its extremity, enabling direct conjugation to targeting moieties via DNA-based linkers. This design supports a simpler and more robust conjugation process, with the potential for improved consistency, faster development cycles, and reduced manufacturing complexity relative to traditional ADC.
How does GenLumina address concerns around silver nanoparticles, including surface plasmon resonance (SPR), photothermal effects, and phototoxicity?
While containing silver, GenLumina’s payload is fundamentally different from conventional silver nanoparticles that give rise to concerns around surface plasmon resonance (SPR), photothermal heating, and non-specific phototoxicity. Our SnB payload consists of few-atom, ultra-small silver-DNA nanoclusters (on the sub-nanometer ) that exist below the size regime required for classical plasmonic behavior. At this scale, silver does not exhibit collective electron oscillations characteristic of larger nanoparticles or aggregates, and therefore does not generate significant photothermal effects upon light exposure. At this size, silver behaves closer to a molecular entity than to a bulk nanoparticle (ref 2). As a result, light activation does not induce bulk heating or non-specific tissue damage, but instead triggers a localized, controlled cytotoxic effect only at the site of activation.
In addition to that, SnB must be present inside a cell to deliver its cytotoxic effect upon light irradiation, further limiting its off-target toxicity. The nanoclusters are too fragile to survive outside of their DNA encapsulation and will immediately dissolve, limiting their toxicity unless the clusters are directly delivered inside the cells. Non-activated molecules will instead form non-toxic salts, which are then excreted from the body according to our pre-clinical results in mice.
Silver is known to accumulate in the body and can be toxic. How does GenLumina address concerns around residual silver exposure and long-term safety?
We recognize historical concerns about silver nanoparticle (AgNPs) toxicity, which depend on particle size, aggregation, surface chemistry, and, most importantly, on the route and duration of exposure. In particular, persistent AgNPs administered intravenously can accumulate in the liver, spleen, and kidneys (ref 3). GenLumina’s approach is designed to mitigate these risks. Our payload is not a persistent or freely circulating nanoparticle, but a targeted, light-activated construct intended for transient local exposure. Preclinical pharmacokinetic studies indicate a short circulating half-life with rapid clearance within hours, rather than prolonged retention or accumulation. In addition, cytotoxic activity is spatially confined to the illuminated field, further limiting off-target exposure and reducing the risk of cumulative silver burden. Ongoing biodistribution, clearance, and toxicology studies are underway to further characterize these parameters in line with regulatory expectations. As reference, historical regulatory assessments cite a human intravenous LOAEL of 0.014 mg/kg/day associated with argyria following chronic silver administration (EPA IRIS, referenced in ICH Q3D/EMA guidance), which provides an external benchmark for evaluating exposure margins (ref 4). GenLumina’s SnB injection contains a silver content of the order of 100 microgram, significantly below published guidelines for silver intake, showing a strong fundamental argument for its use as a safe therapeutic with a broad dosing range.
Light-activated therapies have historically faced limitations in clinical applicability. How does GenLumina ensure sufficient scope and relevance for real-world oncology use?
While we recognize the historical limitations of earlier light-activated modalities such as photodynamic therapy, GenLumina’s approach represents a fundamentally different activation paradigm. Typically, existing PDT suffers from limited efficacy due to a complex biological effect, and a dependence on oxygen inhibiting efficacy in hypoxic tumor regions. GenLumina’s SnB directly targets the DNA in the nucleus, is independent on oxygen, and represents a much more biologically agnostic approach, with 100% efficacy a realistic target.
Here, we benchmark our treatment against clinically approved photodynamic therapies, including Foscan (temoporfin) , a second-generation photosensitizer used in the palliative management of advanced head and neck squamous cell carcinoma (ref 5), and Photofrin (porfimer sodium), a first-generation photosensitizer approved for the treatment of microinvasive endobronchial non-small cell lung cancer (NSCLC) and for palliation of esophageal and obstructing endobronchial NSCLC (ref 6).
EpCAM has historically been a challenging therapeutic target due to off-tumor toxicity. Why is EpCAM viable in GenLumina’s leading program approach?
EpCAM is a well-established marker of cancer stem cells across multiple solid tumors, including colorectal cancer, where EpCAM-expressing cells show enhanced tumor-initiating capacity, chemoresistance, hypoxia tolerance and increased metastatic potential. While biologically validated, EpCAM has been a historically difficult target given its concomitant expression in healthy tissues, especially for systemic therapies due to therapeutic index limitations and toxicities 7. More recent conditional approaches, such as EpCAM-directed Probody programs, have reinforced EpCAM’s importance in epithelial cancers while highlighting the possibility of reducing adverse effects by precision targeting only at the tumor site.
GenLumina takes a different route: EpCAM is used as a local anchoring target, not as a systemic one. Local administration at the tumor site, combined with an EpCAM-binding peptide, enhances tumor confinement and reduces off-tumor exposure. Crucially, cytotoxic activity is triggered only upon
illumination, further restricting the effect to the illuminated field. This allows EpCAM expression in colorectal cancer to be leveraged for precise local control, while minimizing risk to normal EpCAM-positive epithelial tissues.
REFERENCES
1. Abelman et al., Cancers (2023). “Mechanisms of Resistance to Antibody–Drug Conjugates.” doi:10.3390/cancers15041278
2. Shang et al., Nano Today (2011). “Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications.” doi:10.1016/j.nantod.2011.06.004
3. Takáč et al., International Journal of Molecular Sciences (2025). Do We Know Enough About the Safety Profile of Silver Nanoparticles in Oncology? A Focus on Novel Methods and Approaches doi:10.3390/ijms26115344
4. International Council for Harmonisation (ICH). ICH Q3D(R2): Guideline for Elemental Impurities (2022). Silver monograph (p.71).
5. European Medicines Agency (EMA). Foscan (temoporfin): EPAR Summary for the Public (2016).
6. U.S. Food and Drug Administration (FDA). Photofrin® (porfimer sodium) Injection: Prescribing Information (2011).
7. Liu et al., Experimental Hematology & Oncology (2022). “Understanding the versatile roles and applications of EpCAM in cancers: from bench to bedside.” doi:10.1186/s40164-022-00352-4
You can count on us for using our drug to push the boundaries of what is possible in precision oncology.